Its Vapors Are Heavier Than Air
페이지 정보

본문
ChEBI: An ether wherein the oxygen atom is linked to two methyl groups. Dimethyl ether is a colorless gas with a faint ethereal odor. Dimethyl ether is shipped as a liquefied gas beneath its vapor strain. Contact with the liquid may cause frostbite. Dimethyl ether is easily ignited. Its vapors are heavier than air. Nevertheless, inhalation ofexcessive quantities can produce intoxicationand lack of consciousness. Behavior in Fire: Containers might explode. Vapors are heavier than air and will travel long distance to a supply of ignition and flash back. Reactivity with Water No response; Reactivity with Widespread Materials: No reaction; Stability Throughout Transport: Stable; Neutralizing Brokers for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. Within the transportation business, DME is a promising substitute for diesel fuel in buses, trucks, and ships. Its compatibility with present diesel engines and infrastructure, along with lower greenhouse gas emissions, makes dimethyl ether an interesting various for transportation companies seeking to lower their carbon footprint and meet rigorous emissions rules.
The study maps the leading, as effectively because the quickest-growing, regional markets. It further allows stakeholders to identify the important thing country-stage markets inside every region. Porter's 5 forces evaluation help stakeholders in assessing the affect of recent entrants, competitive rivalry, supplier energy, purchaser energy, and the menace of substitution. It helps stakeholders to research the level of competition within the dimethyl ether trade and its attractiveness. Competitive panorama permits stakeholders to grasp their aggressive surroundings and provides an perception into the present positions of key players available in the market.
Dimethyl ether can also be manufactured utilizing natural fuel, which is considered a pricey process. However, the easy availability of natural fuel, pushed by technological developments and excessive analysis and growth investments in the fuel and oil market, is expected to drive the segment’s progress. Topsoe A/S, based in 1940 and headquartered in Denmark, Europe, specialises in providing catalysts, electrolysers, and know-how solutions to decarbonise cement, steel, delivery, chemicals, and aviation sectors. To permit for extra efficient gasification the biomass and coal have numerous preparation processes; similar to chipping of the biomass or producing slurry of the coal. To make sure that the DME produced is clean there are various fuel scrubbing, cleansing and purification processes. DME production from methane can simply use a methane reforming method to provide the syngas for the DME synthesis. The picture under shows a easy representation of the various processes from raw materials to DME. Dimethyl ether is moreover used as a refrigerant. Dimethyl ether may be used as a propellant and solvent in topical pharmaceutical aerosols, and is generally regarded as an basically nontoxic and nonirritant material when used in such functions. However,inhalation of high concentrations of dimethyl ether vapor is harmful. Moreover, pores and skin contact with dimethyl ether liquid might result in freezing of the pores and skin and extreme frostbite.
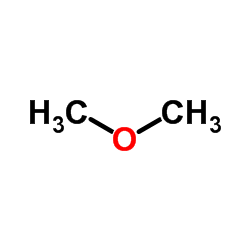
In alcohols, one of the two bonds to the oxygen atom entails hydrogen and one entails carbon. When two or extra carbon atoms are present, nevertheless, isomeric constructions wherein oxygen is bonded to two completely different carbons develop into potential. Such compounds are called ethers. The final formulation for an ether is R—O—R′, where R′ signifies that each R groups need not be the identical. 2. Which What is the common name for CH3OCH3? more soluble in water—ethyl methyl ether (CH3CH2OCH3) or 1-butanol (CH3CH2CH2CH2OH)? 1. Diethyl ether has no intermolecular hydrogen bonding as a result of there isn't any OH group; 1-butanol has an OH and engages in intermolecular hydrogen bonding. 2. Ethyl methyl ether (three carbon atoms, one oxygen atom) is extra soluble in water than 1-butanol (four carbon atoms, one oxygen atom), although both can interact in hydrogen bonding with water.
Ethers are a class of organic compounds that include an sp3 hybridized oxygen between two alkyl groups and have the formulation R-O-R'. Aliphatic ethers have no aryl teams straight connected to the ether oxygen. Aromatic ethers have at the least one aryl ring directly attached to the ether oxygen. In aryl ethers, the lone pair elections on oxygen are conjugated with the aromatic ring which significantly modifications the properties of the ether. The sp3 hybridization of oxygen gives ethers roughly the same geometry as alcohols and water. Dimethyl ether (DME) is a colorless and odorless gas at room temperature and pressure. It is known for its versatility and may create a high-quality mist when released below strain. It may be produced from renewable sources, corresponding to biomass, and has a lower carbon footprint as compared to several standard fuels. As it is a potential vitality provider and a substitute for diesel gas in transportation that assists in decreasing dangerous emissions and bettering the standard of the air, the demand for DME is increasing throughout the globe. Dimethyl ether is often known as methoxy methane, which is an odorless and colorless fuel. Dimethyl ether has a low boiling level and is generated from varied uncooked materials akin to methanol, coal, pure gasoline, and biomass. Dimethyl ether is also a substitute for energy fuels as it has zero sulfur content material. Dimethyl ether has a wide range of purposes in quite a few segments, reminiscent of it is used within the petrochemical and chemical industries as a solvent. The energy and oil business is likely one of the most critical aspects of the global financial system, and the coronavirus occurrence in China in December 2019 & the gradual growth of the illness dramatically decreased crude oil price & demand. Because of the dramatic enhance in COVID circumstances, persons are advised to "sit at dwelling" and refrain from touring.
- 이전글The 10 Worst Milton Keynes Door Panels Failures Of All Time Could Have Been Prevented 24.11.25
- 다음글5 Killer Quora Answers To Asbestos Mesothelioma Lawyers 24.11.25
댓글목록
등록된 댓글이 없습니다.



















